ABSTRACT
The management of sludge is one of the challenging issues that engineers are facing today. In fact, the amount of sludge is the largest byproduct of wastewater treatment. Incineration is one of the most common disposal routes of sludge. However, it generates emissions into the air, soil and water. Thermal applications, such as gasification, offer alternatives for sludge management. The purpose of this paper is to compare incineration and gasification as disposal methods to define their differences, advantages and disadvantages. A literature search was performed in order to establish the comparison categories. Both gasification and incineration are capable of converting hydrocarbon-based hazardous materials to simple, nonhazardous byproducts. However, the conversion mechanisms and the nature of the byproducts differ considerably. Gasification offers significant advantages over incineration, yielding a combustible gaseous product and a solid char product. Gasification has also defined advantages over incineration in terms of the emissions of dioxins, furans, mercury, NOx, N2O, and CO. A significant disadvantage that incineration has over gasification is the cost of air pollution controls for air emissions. Even though gasification is a viable solution for sludge waste management more research is needed in order to obtain the synthesis gas for power production.
Keywords: incineration, gasification, sludge management
1.INTRODUCTION
Digested sludge is a complex mixture of primary mineral grains and fragments of biological and industrial materials. Sewage sludge refers to the residual, semi-solid material left from the treatment of wastewater. The management of sludge plays an important role in wastewater treatment works. Sludge management can be divided into sludge production, treatment, and disposal. Production comprises the quantity and quality of the sludge produced at wastewater treatment plant; treatment comprises the various processes used to change sludge into a form that is acceptable for disposal; and disposal comprises the alternatives for ultimate disposal or utilization of sludge. The application of sewage sludge to agricultural land (which is the most common method of disposal) has become a cause for concern as a threat to human health and the environment. Incineration, which is a common thermal method of disposal, has also become a cause for concern because of its emissions into the air, soil and water. Within the last years, new thermal technologies have arisen in order to get the most of the byproducts of the sludge treatment. One of the thermal technologies that has gained popularity is gasification. Gasification of sewage sludge should produce energy with low emissions, the reduction of sludge to a useable fuel gas and a non- leaching, low-volume, dry residue which is easily to dispose. Both technologies have the potential to produce electricity and/or other products. This is possible because of the sewage sludge composition and its energetic characteristics.
2.SEWAGE SLUDGE COMPOSITION, CHARACTERISTICS AND DISPOSAL METHODS
2.1SLUDGE COMPOSITION AND CHARACTERISTICS
Sludge solids comprise many constituents, and its actual composition can be highly variable according to the type of treatment plant and its methods of operation. In fact, sewage sludge is by far the largest in volume among the byproducts of wastewater treatments. Sludge is the residue generated during the primary (physical and/or
chemical), the secondary (biological) and the tertiary (additional to secondary, often nutrient removal) treatment. It is important to know the sludge composition in order to dispose it effectively. A typical composition and properties of untreated and digested sludge is illustrated in Table 1 (Metcalf, 1991).
Table 1: Typical chemical composition and properties of untreated/digested sludge
| Sludge Composition | Untreated primary | Digested primary | Activated range | ||
| Range | Typical | Range | Typical | ||
| Total dry solids (TS), % | 2.0 – 8.0 | 5.0 | 6.0 – 12.0 | 10.0 | 0.83 – 1.16 |
| Volatile solids (% of TS) | 60 – 80 | 65 | 30 – 60 | 40 | 59 – 88 |
| Ether soluble | 6 – 30 | - | 5 – 20 | 18 | - |
| Ether extract | 7 – 35 | - | - | - | 5 – 12 |
| Protein (% of TS) | 20 – 30 | 25 | 15 – 20 | 18 | 32 – 41 |
| Nitrogen (N, % of TS) | 1.5 – 4 | 2.5 | 1.6 – 6.0 | 3.0 | 2.4 – 5.0 |
|
Phosphorous (P2O5, % of TS) |
0.8 – 2.8 | 1.6 | 1.5 – 4.0 | 2.5 | 2.8 – 11.0 |
| Potash (K2O, % of TS) | 0 – 1 | 0.4 | 0.0 – 3.0 | 1.0 | 0.5 – 0.7 |
| Cellulose | 8.0 – 15.0 | 10.0 | 8.0 – 15.0 | 10.0 | - |
| Iron (not as sulfide) | 2.0 – 4.0 | 2.5 | 3.08 – 8.0 | 4.0 | - |
| Silica (SiO2, % of TS) | 15.0 – 20.0 | - | 10.0 – 20.0 | - | - |
| Alkalinity (mg/L as CaCO3) | 500 – 1,500 | 600 | 2,500 – 3,500 | 580 – 1,100 | |
|
Organic acids (mg/L as Hac) |
200 – 2,000 | 500 | 100 – 600 | 3,000 | 1,100 – 1,700 |
| Energy content | 10,000 – 12,500 | 11,000 | 4,000 – 6,000 | 200 | 8,000 – 10,000 |
| pH | 5.0 – 8.0 | 6.0 | 6.5 – 7.5 | 7 | 6.5 – 8.0 |
It is also necessary to know about the content of heavy metals, pesticides and hydrocarbons in the sewage sludge when is to be incinerated or land filled. A typical metal content in sewage sludge is shown in Table 2 (Fytili and Zabaniotou, 2006).
Table 2: Typical metal content in wastewater sludge
| Metal | Dry sludge (mg/kg) | Metal | Dry sludge (mg/kg) | ||
| Range | Median | Range | Median | ||
| Arsenic (As) | 1.1 – 230 | 10 | Manganese (Mg) | 32 – 9,870 | 260 |
| Cadmium (Cd) | 1 – 3.410 | 10 | Mercury (Hg) | 0.6 – 56 | 6 |
| Chromium (Cr) | 10 – 99,000 | 500 | Molybdenum (Mo) | 0.1 – 214 | 4 |
| Cobalt (Co) | 11.3 – 2,490 | 30 | Nickel (Ni) | 2 – 5,300 | 80 |
| Copper (Cu) | 84 – 17,000 | 800 | Selenium (Se) | 1.7 – 17.2 | 5 |
| Iron (Fe) | 1,000 – 154,000 | 17,000 | Tin (Sn) | 2.6 – 329 | 14 |
| Lead (Pb) | 13 – 26,000 | 500 | Zinc (Zn) | 101 – 49,000 | 1700 |
Total concentration of heavy metals in sewage sludge indicates its extent of contamination and restricts the sludge disposal methods. Concentrations of zinc (Zn), copper (Cu), nickel (Ni), cadmium (Cd), lead (Pb), mercury (Hg) and chromium (Cr) are principal minor elements of sludge and one of the reason that the use of sludge for agricultural purposes has been restricted. There have been many experimental works in order to determine the extractable trace metals in sludge, even though methods used have not been unreservedly accepted by the scientific community.
A crucial characteristic of sewage sludge, when a thermal application is considered as a disposal method, is its energy (thermal) content or heating value. This property is used, as the priority, for evaluating the potential of sewage sludge. Regarding the empirical approach, there are three types of models that are normally used to predict heating values based on the following analyses: (1) physical or chemical compositions, (2) proximate analysis and (3) ultimate analysis. Typical heating values of sewage sludge are illustrated in Table 3. Table 3 shows the results of a prediction of heating value using the proximate and ultimate analysis (Kitiyanan et al., 2006). The sludge energy content value plays an important role in design calculations and economic analysis. As seen in Table 3, energy content of sewage sludge may vary from 12,640-20,900 kJ/kg.
Table 3: Heating values (HV) of sewage sludge from literature compared to calculated values
| Sludge Sample | HVa (kJ/kg) | HVb (kJ/kg) | % error | HVc (kJ/kg) | % error |
| 1 | 16,558 |
15,919 |
-3.9 | 16,365 | -1.2 |
| 2 | 20,900 |
19,419 |
-7.1 | 20,598 | -1.4 |
| 3 | 17,140 |
16,737 |
-2.3 | 17,896 | 4.4 |
| 4 | 16,774 |
15,533 |
-7.4 | 16,363 | -2.4 |
| 5 | 16,564 |
15,612 |
-5.7 | 16,735 | 1.0 |
| 6 | 13,343 |
12,280 |
-8 | 12,755 | -4.4 |
| 7 | 16,611 |
13,819 |
-16.8 | 14,549 | -12.4 |
| 8 | 12,790 |
12,988 |
1.6 | 13,100 | 2.4 |
| 9 | 12,640 |
12,115 |
-4.1 | 12,068 | -4.5 |
| 10 | 18,440 |
17,986 |
-2.5 | 17,545 | -4.9 |
a HV from literature, b HV calculated using proximate analysis, c HV calculated using ultimate analysis
2.2SLUDGE DISPOSAL METHODS
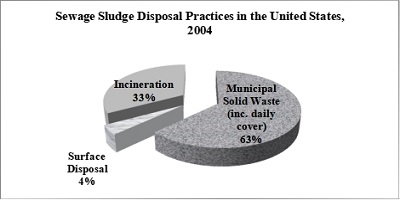
In recent years, methods formerly used for the disposal of sewage sludge, including landfill, incineration, ocean dumping and disposal on agricultural land have become much less acceptable. Ocean dumping of sewage sludge was outlawed in the United States (US) since 1998. Space for agricultural land disposal is not available in many urban areas and is meeting with increased opposition from farmers largely because of concern over potential heavy metal contamination from sludge. Most of the US sewage sludge that is not applied to soils goes to municipal solid waste landfills, as shown in Figure 1. Thirty-three percent of the US sewage sludge has been incinerated. Dedicated surface disposal units, also known as monofills, handle only a small percentage of the nation’s sewage sludge (NEBRA, 2007). Although agriculture and landfill are the disposal methods of biosolids that are most used in the US (36 percent and 38 percent, respectively) (UNEP, n.d), incineration and gasification are part of the sludge disposal methods that use thermal processes to obtain more of its byproducts and are able to provide a solution in terms of volume reduction.
The consideration of thermal applications as a sludge waste management solution is of increasing importance today. Incineration of sewage sludge is perhaps the most established thermal application as a disposal method of sludge. There are approximately 170 sewage sludge incineration plants in operation in US (UNEP, n.d.). The design of an incineration process includes three types of incinerators: multiple hearth, fluidized bed, and electric infrared. Over 80 percent of the identified operating sludge incinerators are of the multiple hearth design; about 15 percent are fluidized bed combustors, and 3 percent are electric (EPA, 1995). The remaining combustors co-fire refuse with sludge (EPA, 1995). Incineration involves the firing of sewage sludge at high temperature in an enclosed structure. This process reduces the sludge to a mass of ash that is less than 20 percent of its original volume. Sewage sludge incineration has been a solution for urban areas where lack of disposal space is present and eliminates some environmental and health problems by destroying pathogens and toxic organic chemicals. Modern incineration configuration includes the use of heat produced by sludge combustion to heat water that becomes steam. Produced steam can turns a turbine to generate electricity.
Figure 1: Sewage Sludge Disposal Practices in the United States, 2004
Another thermal process used as a disposal method of sludge is gasification. The Gasification Technologies Council has defined gasification as a process that converts carbonaceous materials through a process involving partial oxidation of the feedstock (i.e. sewage sludge) in a reducing atmosphere in the presence of steam at temperatures sufficient to convert the feedstock to synthesis gas (syngas). Indeed, gasification converts inorganic matter in the feedstock (when it is a solid or semi-solid) to a glassy solid material known as vitreous frit or slag and halogens into corresponding acid halides. In the early 1990’s emerged a big interest in this process because of the increasing performance of gas turbines for the production of electricity. The importance of gasification is that gas produced could replace natural gas in the generation of electricity. Cost studies has shown gasification is one of the thermal application that less consume energy, although, an energy input is necessary to start the process (Ferrasse et al., 2003).
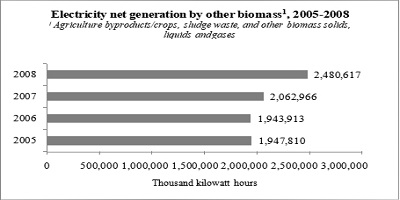
There are no ready data that show the percentage of sewage sludge that has been treated by gasification/incineration for electricity generation purposes. However, data from the US Department of Energy show an increase of 17 percent of net electricity generation by other biomass that includes sludge waste, as illustrated in Figure 2. This may suggest that using thermal processes as a sludge waste management solution has been increasing over time.
Figure 2: Electricity net generation by other biomass1, 2005-2008
3.THERMAL PROCESSES IN SLUDGE WASTE MANAGEMENT
Technologies for the thermal treatment of sewage sludge are appraised with reference to their efficacy in terms of
(a) operational parameters, (b) pre and post-treatment requirements, and (c) the extent of their use for the application (Furness et al., 2000). There are several thermal processes in sludge treatment that may include incineration, wet oxidation, thermophilic aerobic digestion, gasification, and others. Although there are several options available in sludge waste management using thermal processes, only incineration and gasification will be discussed in order to compare their advantages and disadvantages in terms of process operation, byproducts and emissions.
3.1INCINERATION
There are different types of process in incineration. Multiple hearth and fluidized bed furnaces are the most popular, and will be discussed moreover. The difference between those two types of furnaces is that multiple hearth furnaces usually burn mechanically dewatered (wet) sludge, while fluidized bed furnaces can burn both wet and semi-dried sludge. Other processes may include electric, single hearth cyclone, rotary kiln, and wet air oxidation.
The multiple hearth furnace (MHF) is a vertically oriented cylinder. The dimensions of the MHF are normally about 26 ft. in diameter and 46 ft. of total height consisting of 14 hearths. The sludge is fed at the top and is transported from hearth to hearth by scrapers, which are attached to a rotating shaft. MHF can be divided into three operational zones: (1) drying zone, (2) sludge combustion and (3) cooling zone. The upper hearths comprise the drying zone where most of the moisture in the sludge is evaporated. Temperatures in the drying zone are between 425 and 760⁰C (800 and 1400⁰F). In the middle hearths sludge combustion takes places as the temperature is increased to about 925⁰C (1700⁰F). The third zone is the cooling zone, which is made up of the lowermost hearth(s). Ash is cooled in this zone as its heat is transferred to the incoming combustion air. Disadvantages of MHF are related to the cost associated with the frequent need for supplementary fuel to sustain the incineration process. The use of scrubbers, cyclones and dry cyclone are usually employed to control MHFs’ emissions.
Fluidized bed combustors (FBCs) consist of a vertically oriented outer shell constructed of steel and nozzles that are designed to deliver blast of air. Combustion of the sludge can be divided in two zones. In zone 1, evaporation of the water and pyrolysis of the organic materials occur nearly simultaneously as the temperature of the sludge raise rapidly between 750 to 925⁰C (1400 to 1700⁰F). The second zone functions as an afterburner where the remaining free carbon and combustible gases are burn. Different types of scrubber as venturi scrubbers or venturi/impingement tray scrubber usually control FBCs’ emissions. FBCs are able to achieve complete combustion with 20 to 50 percent excess air that is less the excess air required by MFH and usually have lower fuel requirements as compared with MFH.
Sewage sludge incinerators potentially have significant air emissions. The major pollutants emitted are: (1) particulate matter, (2) metals, (3) carbon monoxide (CO), (4) nitrogen oxides (NOx), (5) sulfur dioxide (SO2), and
(6) unburned hydrocarbons. If partial combustion of sludge is present in the incinerator’s operations it can result in emissions of intermediate products of incomplete combustion, including toxic organic compounds.
3.2GASIFICATION
During gasification process, sewage sludge undergoes through physical and chemical changes. The chemical reactions take place in the presence of steam in an oxygen-lean reducing atmosphere. Chemical reactions can be described by the equations illustrated in Table 4.
Table 4: Chemical reactions during gasification of sludge
| Chemical reaction | Thermal process |
| C (fuel) + O2 → CO2 + heat | Exothermic |
| C + H2O (steam) → CO + H2 | Endothermic |
| C + CO2 → 2CO | Endothermic |
| C + 2H2 → CH4 | Exothermic |
| CO + H2O → CO2 + H2 | Exothermic |
| CO + 3H2 → CH4 + H2O | Exothermic |
The gasification of sludge can be divided in three stages that are: (1) drying, (2) pyrolysis and (3) thermal. In the drying stage, water content of sludge is removed as moisture. The dry solid content output from the drier is between 85-93 percent of dry solids depending on the type of gasifier installed (Furness and Hoggett, 2000). This stage takes place in a temperature around 150⁰C (302⁰F). Once the sludge is practically dried, its temperature is raised to 400⁰C (752⁰F), which is when pyrolysis occurs (second stage). In the final stage, the pyrolysis products, condensable and non-condensable vapors and char undergo gasification (thermal stage), where they are oxidized and then reduced and transformed into char, steam, tar and gases. Sludge is oxidized with a sub-stoichiometry oxygen input. The thermal stage occurs at the temperature between 800 to 1400⁰C (1472 to 2552⁰F). Supplementary fuels such as coal or petroleum coke are necessary to maintain the desired gasification temperatures in the reactor.
After the thermal stage, the raw synthesis gas temperature is reduced by quenching with water, slurry, and/or cool recycled syngas. Further cooling may be done by heat exchange in a syngas cooler before entrained particulate is removed. Particulate matter is captured in the water and filtered from the water if direct-water scrubbing is utilized. Alternatively, particulates may be removed via dry filtration or hot gas filtration. Moisture in the syngas condenses as it is cooled below its dewpoint. Any particulate scrubber water and syngas cooling condensates contain some water-soluble gases (NH3, HCN, HCl, H2S). Further refinement of the syngas is conditional upon the end use of the product syngas but usually includes the removal of sulfur compounds (H2S) for the recovery of high-purity sulfur as a marketable product. The various water streams resulting from syngas cooling and cleaning are typically recycled to the gasifier or to the scrubber after entrained solids have been removed. A small portion of the water must be purged from the system to avoid accumulation of dissolved salts. The resulting water is then recycled to the process or a portion blown down to a conventional waste water treatment system. Gas condensate may also be steam-stripped to remove ammonia, carbon dioxide, and hydrogen sulfide. All stages take place in a gasification reactor which its operation varies depending its type.
The most frequent reactors used for gasification are (1) fixed bed reactor, (2) fluid bed reactor and (3) circulating (moving) bed reactor. Fixed-bed and fluidised-bed gasifiers are usually refractory-lined or water-cooled to protect the reaction chamber from high temperatures, and have a rotary or fixed grate. Moving-bed gasifiers are generally indirectly heated through a metal reaction chamber and are less common than fixed or fluidised beds. Although one of the most recent developments in the application of thermal gasification to sewage sludge is the Lurgi- Rhurgas process based on a circulating fluidized bed. This thermal process may produce a gas having a calorific value of about 23 MJ/m3, which is gained from the increased contact brought by the recirculating fluidised media.
Only limited data on emissions from sewage sludge gasification is available. This is reasonable because emissions are highly variable and depend on gasifier type, sludge characteristics, process conditions (temperature and pressure) and gas conditioning systems. What has been defined is that emissions of dioxins, furans, mercury and other heavy metals, NOx, N2O, and CO are reduced significantly. Although big concerns exist with the slag produced that may still have concentration of heavy metals. Organic compounds such as benzene, toluene, naphthalene, and acenaphthalene have been detected at very low levels in the produced gas from some gasification systems. However, when the gas is used as a fuel and combusted in a gas turbine, the emissions of these compounds or other organic hazardous air pollutants are either not detected or present at sub-part-per-billion
concentrations in the emitted stack gas. The supplementary fuel used to maintain desired temperatures in the reactor do not increase emissions of CO2, hence, contributes to the production of more gas.
4.COMPARISON OF SLUDGE TREATMENT BY GASIFICATION VS. INCINERATION
Both gasification and incineration are capable of converting sewage sludge to simple, nonhazardous byproducts. However, the conversion mechanisms and the nature of the byproducts differ considerably, and these factors should justify the separate treatment of these two technologies in the context of environmental protection and economics. Based on the information compiled, gasification and incineration were compared into four broad categories: (1) sludge preparation and feeding, (2) operation, (3) gas cleanup and (4) byproducts handling. Table 5 details the differences found considering the four aforementioned categories.
Table 5: Differences between incineration vs. gasification
| Category | Incineration | Gasification |
| Sludge preparation | 1. Sludge can be wet or semi-dried. It depends of the type of furnace(MHF/FCB). | 1. Sludge water content is removed as moisture. It needs to be dried. |
|
Process operation |
|
|
| Gas cleanup and uses |
|
|
|
Byproduct handling |
treatment plant. |
|
The sludge preparation for both processes includes a drying step, where sludge water content is removed. Although, incineration can bring the option of burning semi-dried sludge if a fluid bed furnace is part of its process. Gasification and incineration technologies are significantly different in terms of byproduct utilization and treatment. Slag is the primary solid byproduct of gasification and the quantity produced is a function of how much mineral matter is present in the sludge. On the other hand, incineration has a byproduct that has been used as daily cover for landfill but several concerns have arisen in terms of ash toxicity, as mentioned before.
Based on the differences of both technologies, advantages and disadvantages can be defined. A summary of the advantages and disadvantages of the technologies are illustrated in Table 6.
Table 6: Advantages and disadvantages of incineration vs. gasification
| SludgeTreatment Method | Advantages | Disadvantages |
|
Incineration |
|
|
|
Gasification |
|
|
Both technologies offer the same advantages in terms of volume and weight reduction of sludge and less dependency of landfill and landspreading as final disposal methods. However, the production of a syngas is one of the most attractive complement of gasification because of its uses. The disadvantage that incineration has over gasification is the cost related to air pollution controls. A significant advantage that gasification has is that claim to be able to meet existing, and possible future, emission standards. In contrast with incineration that may be even more costly because of future emission legislation.
Incineration has been a well known technology over time, even though gasification is not a new technology at all. However, few studies have been conducted on sewage gasification (Fytili and Zabaniotou, 2006). More fundamental research, on the effect of sludge in gasification, need to be attempted, in order to obtain the desirable syngas for power production and/or the other products. There are several publications about gasification where the use of sewage sludge is mentioned, although it is included as part of biomass gasification that may include other materials as wood and municipal solid wastes.
5.CONCLUSIONS
Sludge is the residue generated during the primary, the secondary and the tertiary treatment. Technologies for the thermal treatment of sewage sludge are being used over time as final disposal methods of sludge. A crucial characteristic of sewage sludge, when a thermal application is considered as a disposal method, is its energy (thermal) content or heating value. This property is use, as the priority, for evaluating the potential of sewage sludge.
Two thermal applications were compared in order to establish their differences, advantages and disadvantages. Incineration involves the firing of sewage sludge at high temperature in an enclosed structure and has been a solution for urban areas where lack of disposal space is present. Modern incineration configuration includes the use of heat produced by sludge combustion to heat water that becomes steam. Produced steam can turns a turbine to generate electricity. However, operation and maintenance costs are high because of air pollution controls that must be included in an incineration facility. On the other hand, gasification is a thermal application that offers advantages over incineration. The importance of gasification is the syngas produced that could replace natural gas in the generation of electricity. Cost studies have shown gasification is one of the thermal applications that less consume energy, although, an energy input is necessary to start the process. In addition it has been shown that emissions of dioxins, furans, mercury and other heavy metals, NOx, N2O, and CO are reduced significantly.
Both gasification and incineration are capable of converting sewage sludge to simple, nonhazardous byproducts. However, sludge gasification should produce energy with low emissions and the reduction of sludge to a useable fuel gas and a non-leaching, low-volume, dry residue which can be easily disposed or reused. In addition, gasification has the potential to provide a solution to recent and possible future, sludge disposal problems and management legislation. More fundamental research on the effect of sludge in gasification is needed, in order to obtain the desirable syngas for power production or other prod
Post time: Mar-28-2022